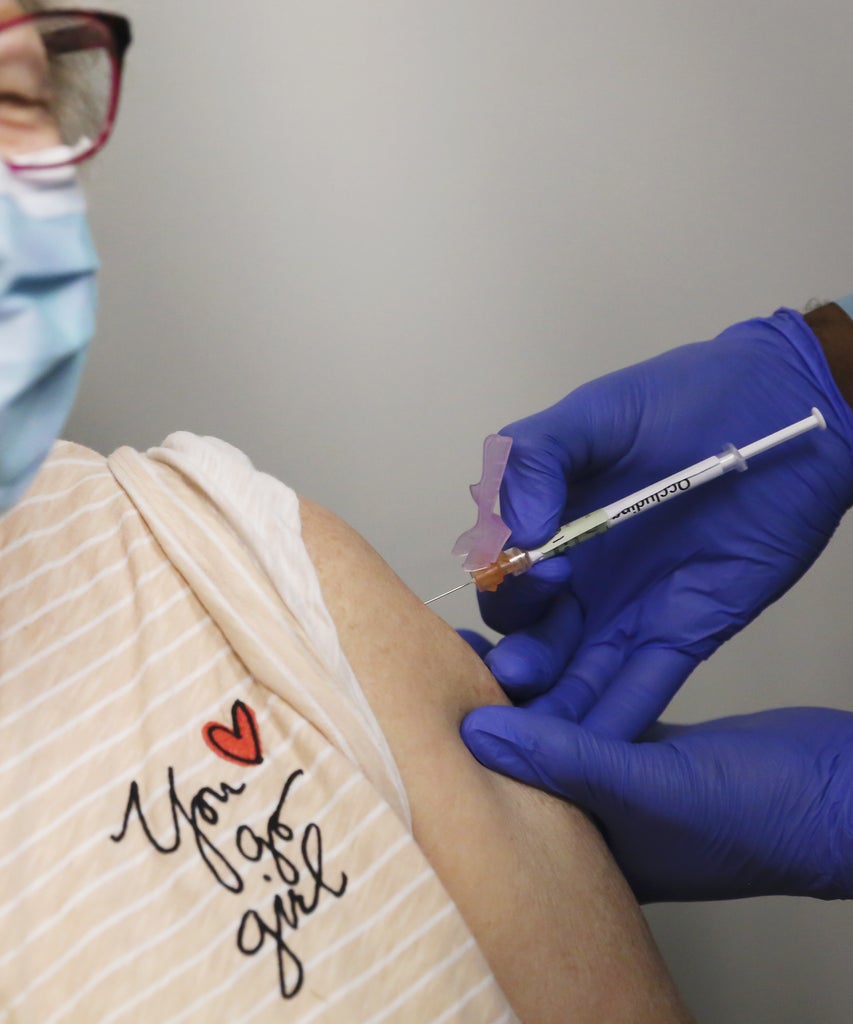
The Food and Drug Administration (FDA) approved the Moderna vaccine for emergency use shortly before 8 p.m. Eastern Time on Friday, Dec. 18. The news comes just one week after the FDA approved the Pfizer/BioNTech vaccine for mass distribution across the U.S.
“With the availability of two vaccines now for the prevention of COVID-19, the FDA has taken another crucial step in the fight against this global pandemic that is causing vast numbers of hospitalizations and deaths in the United States each day,” FDA Commissioner Stephen M. Hahn, MD, said in an FDA press release. “Through the FDA’s open and transparent scientific review process, two COVID-19 vaccines have been authorized in an expedited timeframe while adhering to the rigorous standards for safety, effectiveness, and manufacturing quality.”
Today, FDA issued an emergency use authorization (EUA) for the second vaccine for the prevention of #COVID19 caused by SARS-CoV-2. The emergency use authorization allows the vaccine to be distributed in the U.S for use in individuals 18 years and older. https://t.co/w4BQVg0n2G pic.twitter.com/cw5JwYqxsh
— U.S. FDA (@US_FDA) December 19, 2020
Yesterday, an FDA advisory panel recommended that the Moderna COVID-19 vaccine be cleared for use in people 18 years of age and older. They voted 20-0 to recommend use of the vaccine, with one abstention. “The evidence for the vaccine highly outweighs any issues that we have seen,” Hayley Gans, a member of the FDA panel and a Stanford University pediatrics professor, said of the Moderna vaccine yesterday.
Federal officials have said that they’ll distribute nearly six million doses of the Moderna vaccine across the nation, likely within the next 24 hours, The Wall Street Journal reports. Each state will have the final say on how to distribute the vaccine, but the Centers For Disease Control and Prevention (CDC) recommends that frontline health care workers and nursing homes get the vaccine first. Most states are following this advice. There’s currently not enough data to recommend the vaccine to minors and people who are breastfeeding or pregnant.
Both the Pfizer and Moderna vaccines are made with modified messenger RNA (MRNA) and both require two doses; the doses of the Moderna vaccine are given 28 days apart, while Pfizer’s are given 21 days apart. After reanalyzing data from Moderna, the FDA confirmed that this vaccine was 94% effective two weeks after the second dose; Pfizer’s was found to be 95% effective. “Which one is better? The one you can get first,” says Jill Grimes, MD, a board-certified family physician in Texas.
The major upside of the Moderna vaccine is that it can be more widely distributed than the Pfizer shot because it can be stored at standard refrigerated temperatures of 36° to 46°F for 30 days, Moderna noted in a company press release. Meanwhile, the Pfizer vaccine must be kept at minus-94 degrees Fahrenheit and therefore must be packed in dry ice to be distributed. This makes Moderna’s shot a good option for hospitals and clinics in rural areas that might not have the equipment to store Pfizer’s vaccine properly. “Smaller venues including community centers, nursing homes, community pharmacies, and more remote areas can benefit from the Moderna vaccine because storage is simple,” says Preeti N. Malani, MD, chief health officer at the University of Michigan.
The side effects of both the Pfizer and the Modern vaccine are reportedly mild, says Amesh Adalja, a senior scholar at Johns Hopkins Center for Health Security. He notes that it’s hard to know how the side effects of the two vaccines will compare, given that no study has compared the two directly. “[With Moderna], some people will get injection site soreness, aches, and pains, but it’s preferable to having COVID-19,” says Dr. Adalja, who received his first injection of the Pfizer vaccine today at his hospital and said he felt fine after taking it.
The FDA noted that in a trial of Moderna’s vaccine, a few participants experienced more moderate side effects. One person experienced severe nausea and vomiting; two others reported facial swelling. Both people who experienced swelling previously had cosmetic dermal fillers. The FDA’s Moderna briefing document noted that there were three reports of Bell’s palsy, a form of temporary facial paralysis, among those who received the vaccine as well as one case in the group of study participants who received the placebo. (The Pfizer vaccine trial had four cases in the vaccine group.) This number of cases wasn’t statistically significant and was on par with the rate of this condition in the general population, Rachel Zhang, an FDA medical officer, told the advisory panel Thursday.
Emergency use authorization is granted only in instances when there’s a major public health threat with no fully approved treatment or vaccine. The only person to abstain from granting the EUA on the FDA advisory panel was Michael Kurilla, MD, PhD, the director of the Division of Clinical Innovation at NIH’s National Center for Advancing Translational Sciences. In a statement sent to Refinery29, he noted that he abstained because of the way the FDA worded the question he was voting on, and because of a lack of long-term safety data. “I felt uncomfortable with an assessment of the vaccine’s risk/benefit profile without also including consideration of the individual’s COVID-19 disease risk,” he added.
The rest of the panel, along with many doctors and experts, agree that granting the EUA is the right choice. “On balance, this vaccine’s benefits greatly outweigh the risks of COVID-19, especially given the present danger and public health crisis,” Dr. Adalja says.
The Moderna approval comes at the end of a devastating week, during which the U.S. broke its record for total deaths in a single day with 3,600 deaths on Wednesday, Dec 16. “Both vaccine approvals really represent a moment of hope, even though the COVID numbers are really terrible right now,” says Dr. Malani. “This is a great moment, but I hope it’s also a moment where we can say, ‘I can do this, I can limit my gatherings, I can wear a mask, I can stay home if I’m sick, and I can do the holidays differently.’ This is the first time there’s really felt like an end in sight.”
For now, you can learn more information about when you might be able to receive the vaccine in your state by checking the Vaccine Allocation Planner for COVID-19 website. Until then, and following your vaccination, continue following the CDC’s guidelines for preventing the spread of COVID-19, including wearing masks, washing your hands, and staying distanced.
Like what you see? How about some more R29 goodness, right here?
Why Is Mike Pence Getting Vaccinated On TV?
I'm Obsessed With Watching Nurses Get Vaccinated
A COVID-19 Vaccine Got Emergency U.S. Approval
from Refinery29 https://ift.tt/2LRpC3N
via IFTTT
